Targeted Lung Denervation for COPD
Chronic obstructive pulmonary disease (COPD) is a chronic inflammatory lung disease. With COPD, changes in the airways block the normal flow of air in and out of the lungs. This causes a variety of symptoms, including:1,2
- Persistent coughing
- Wheezing
- Chest tightness
- Excess mucus production
Drugs like inhalers, steroids, and antibiotics are often used to treat COPD. However, many people still have flare-ups while taking these medicines. To better treat COPD, researchers are exploring a new procedure called targeted lung denervation (TLD). While it is still being studying in clinical trials, TLD shows promising results for safely treating moderate to severe COPD.1
What is targeted lung denervation?
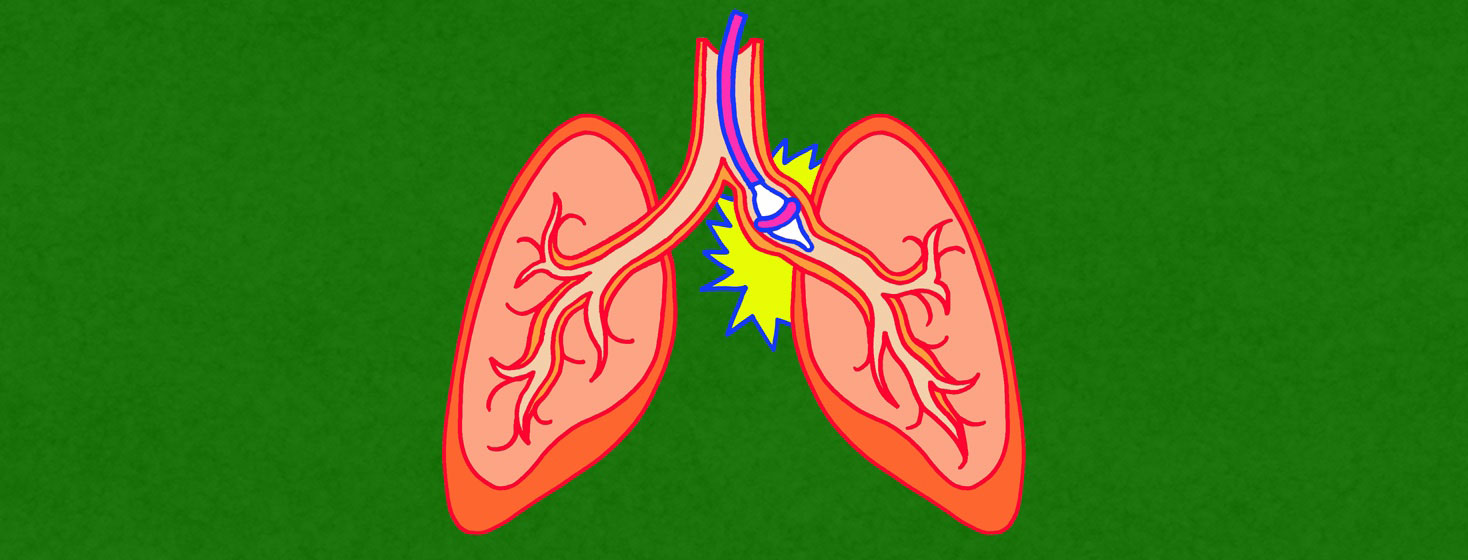
Targeted lung denervation (TLD) is a one-time, non-surgical, outpatient procedure. The procedure is designed to reduce airway nerve activity.1,3
During the procedure, a doctor inserts a thin, flexible tube through the mouth into the main airways of both lungs. Once in place, the doctor inserts a small balloon through the tube and uses it to inflate the airways.1-3
An electrode then delivers targeted radiofrequency (RF) to the nerves on the outside of the airways. The tube is rotated, and the electrodes deliver RF several times to interrupt the nerve signals.1-3
How does targeted lung denervation help manage COPD?
When we breathe, airflow in our lungs is controlled by communication along a nerve pathway that links our lungs and brain. Research shows that in people with COPD, the nerves located on the outside of the airways are overactive. In turn, these tell the brain and lungs to increase levels of mucus and tighten the airways. This makes breathing more difficult.1-3
COPD drugs work by temporarily blocking some of these nerve signals to keep airways open and mucus levels down. While these drugs can help with COPD symptoms, they do not always effectively control flare-ups.1-3
TLD disables some of the nerves located outside of the airways. This prevents them from sending an excess of signals that increase mucus and tighten the airways. This reduces the risk of COPD flare-ups.1-3
One study looked at 46 people who received TLD. Researchers looked at the long-term impact of TLD COPD flare-ups, lung function, and quality of life over 3 years. The rate of people having at least 1 moderate or severe COPD flare-up included:1
- 70 percent at the 1-year follow-up
- 61 percent at the 2-year follow-up
- 46 percent at the 3-year follow-up
Lung function and quality of life remained stable over 3 years of follow-up visits. This is a promising finding since most research shows that both lung function and quality of life decline as COPD progresses.1
Possible side effects
Current data from clinical trials shows that there are few side effects of TLD.
In one study, 9 people experienced gastrointestinal problems in the first year after the procedure. The main issue was impaired gastric emptying, which is when the stomach does not empty food as quickly as it should. These problems resolved by the second and third-year follow-up visits.1
While studies so far have yet to find any major side effects of TLD, more research needs to be done on larger groups of people. As of early 2021, researchers are still studying TLD in several clinical trials.
Things to know
Since TLD is still being studied in clinical trials, the procedure is not widely available. The Airflow-3 trial is currently recruiting people with moderate to severe COPD who have significant symptoms and a history of flare-ups. The trial is expected to end in 2027.3
If you are interested in participating in a clinical trial for TLD, talk to your doctor. Clinical trials may not be right for everyone, and they have strict enrollment criteria. Your doctor can help you decide if this is a good option for you.
Join the conversation